About us
LOCOMOTION - ‘LOng COvid Multidisciplinary consortium: Optimising Treatments and servIces acrOss the NHS’
Long COVID and the LOCOMOTION Study
The LOCOMOTION study will identify best practice in providing Long COVID (LC) services. Ensuring people are supported quickly and receive the right treatments from the right healthcare professionals. Whether this be in their own home, through their GP, or at specialist LC clinics.
LC refers to a set of symptoms that persist for longer than four weeks after contracting coronavirus. LC has many symptoms, including breathlessness, fatigue, and brain ‘fog’. It has the potential to deepen health inequalities and is having a disproportionate impact on patients with existing health conditions and people from lower income backgrounds.
Contents of this page:
Aims of LOCOMOTION
The researchers aim to deliver several outcomes from the research, including:
- An evaluation of treatment options. The researchers will produce best practice guidance on which are the most effective treatments. This guidance will be rapidly shared with specialist LC clinics across the four home nations, to standardise care.
- Investigating how many people are unable to work because of LC. The researchers will look at developing a vocational rehabilitation programme to get LC patients back into employment.
- The study will also look to identify if there are physical, emotional, and cognitive triggers that can result in people experiencing symptoms which set their recovery back. They will investigate whether these can be self-managed at home.
![]()
The research will take place in three settings. Through NHS LC clinics and GP surgeries, we will track where patients are being referred or not referred to and learn from the experience of clinics by interviewing patients and recording outcomes. Within the home, people with LC will self-monitor on a mobile device, using a set of questions about their symptoms which are built into an application on the device.
Throughout the study, specialists in ‘Healthcare Inequality’ will reach people who are not accessing clinics for a variety of reasons. We will put in place new processes in clinics and doctors’ surgeries which will be monitored throughout to ensure they are the correct standard, accessible for patients and staff, and cost-effective.
LOCOMOTION Work Packages
Each workstream of the study contains multiple 'tasks', which are also referred to as 'work packages'. These are outlined in the graphic below.
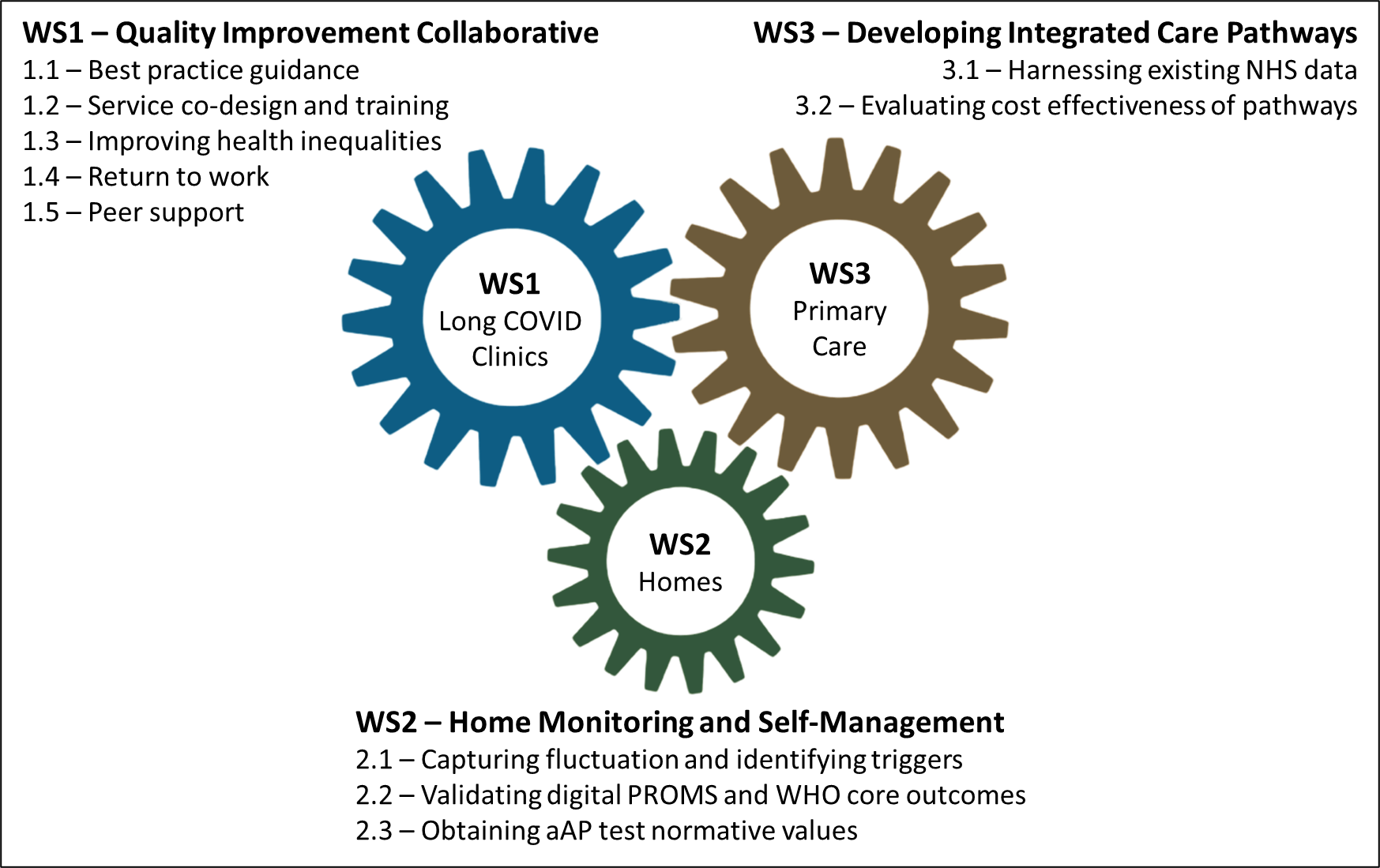
Read our Protocol Paper to learn more about each work package.
The Study Team
LOCOMOTION aims to cover a large geographical area across the UK, working with participants from a wide range of backgrounds. The project team consists of clinical sites, university partners, patients and the public, and technology partners, all working collaboratively. Further information about each of these groups is detailed below and on their individual pages.
The project is led by the University of Leeds. The joint Chief Investigators of the project are Manoj Sivan and Brendan Delaney.
![]()
Clinical Sites
The research is based on the experience of a wide range of NHS professionals already treating people in ten LC clinical sites across the UK. We have a diverse range of clinical sites, with representation from Scotland, Wales, and across England. Visit the Clinical Sites page for more information.
University Partners
The study is led by academics from universities with links to other LC funded studies. Visit the University Partners page for more information.
Patient and Public Involvement
LOCOMOTION has been developed alongside LC patients and will continue to include patients working as equal partners. These patients form our Patient Advisory Group (PAG). We also have a Patient Advisory Network (PAN) and an External Advisory Group (EAG) which also include patients and the public. Visit the Patient and Public Involvement page for more information.
Technology Partners
Athena CX – A platform to create and distribute experience sampling studies directly to participants phones. Integrated with 3rd party devices such as 'Fitbit' to also collect data from wearables too. This platform us being used in our work package 2.1 'Capturing fluctuation and identifying triggers'.
ELAROS – Developers, in collaboration with Pipe and Piper Ltd, of the clinical web portal and patient app that hosts the C19-YRS questionnaire. This web portal/app is being used for data collection in our work package 2.2 'Validating digital PROMs and WHO core outcomes'.
Collaborators
Prof. Christopher Mathias at Queen Square Autonomic Unit, University College London – Developer of the adapted Autonomic Profile (aAP). The at-home test of autonomic dysfunction, that is being used in our work package 2.3 'Obtaining aAP test normative values'.
